Publications
A Kalirin Missense Mutation Enhances Dendritic RhoA Signaling and Leads to Regression of Cortical Dendritic Arbors Across Development
In Press 9/20/21 - Proceedings of the National Academy of Sciences of the United States of America

Normally, dendritic size is established prior to adolescence then remains relatively constant into adulthood due to a homeostatic balance between growth and retraction pathways. However, schizophrenia is characterized by accelerated reductions of cerebral cortex gray matter volume and onset of clinical symptoms during adolescence, with reductions in layer 3 pyramidal neuron dendritic length, complexity, and spine density identified in multiple cortical regions postmortem. Nogo receptor 1 (NGR1) activation of the GTPase RhoA is a major pathway restricting dendritic growth in the cerebral cortex. We show that the NGR1 pathway is stimulated by OMGp and requires the Rho guanine nucleotide exchange factor, Kalirin-9 (KAL9). Using a genetically encoded RhoA sensor, we demonstrate that a naturally occurring missense mutation in Kalrn, KAL-PT, that was identified in a schizophrenia cohort, confers enhanced RhoA activitation in neuronal dendrites compared to wildtype KAL. In mice containing this missense mutation at the endogenous locus there is an adolescent-onset reduction in dendritic length and complexity of layer 3 pyramidal neurons in the primary auditory cortex. Spine density per unit length of dendrite is unaffected. Early adult mice with these structural deficts exhibited impaired detection of short gap durations. These findings provide a neuropsychiatric model of disease capturing how a mild genetic vulnerability may interact with normal developmental processes such that pathology only emerges around adolescence. This interplay between genetic susceptibility and normal adolescent development, both of which possess inherent individual variability, may contribute to heterogeneity seen in phenotypes in human neuropsychiatric disease.
Grubisha, MJ, Sun T, Erickson SL, Eisenman L, Chou S, Helmer CD, Trudgen MT, Ding Y, Homanics GE, Penzes P, Wills ZP, Sweet, RA
Cover art to be submitted!
Amyloid Beta Peptides Block New Synapse Assembly by Nogo Receptor Mediated Inhibition of T-Type Calcium Channels
October 12, 2017 - Neuron
Compelling evidence links amyloid beta (Abeta) peptide accumulation in the brains of Alzheimer’s disease (AD) patients with the emergence of learning and memory deficits; yet a clear understanding of the events that drive this synaptic pathology are lacking. We present evidence that neurons exposed to Abeta are unable to form new synapses, resulting in learning deficits in vivo. We demonstrate the Nogo receptor family (NgR1-3) act as Abeta receptors mediating an inhibition of synapse assembly, plasticity and learning. Live imaging studies reveal Abeta activates NgRs on the dendritic shaft of neurons triggering an inhibition of calcium signaling. We define T-type calcium channels as a target of Abeta-NgR signaling, mediating Abeta’s inhibitory effects on calcium, synapse assembly, plasticity and learning. These studies highlight deficits in new synapse assembly as a potential initiator of cognitive pathology in AD, and pinpoint calcium dysregulation mediated by NgRs and T-type channels as key components.
Yanjun Zhao, Sivaprakash Sivaji, Michael C. Chiang, Haadi Ali, Monica Zukowski, Sareen Ali, Bryan Kennedy, Alex Sklyar, Alice Cheng, Zihan Guo, Alexander K. Reed, Ravindra Kodali, Jennifer Borowski, Georgia Frost, Patrick Beukema and Zachary P. Wills
Cover Art Submission (not chosen but too cool to leave out!)
The Nogo Receptor Family Restricts Synapse Number in the Developing Hippocampus
February 9, 2012 - Neuron
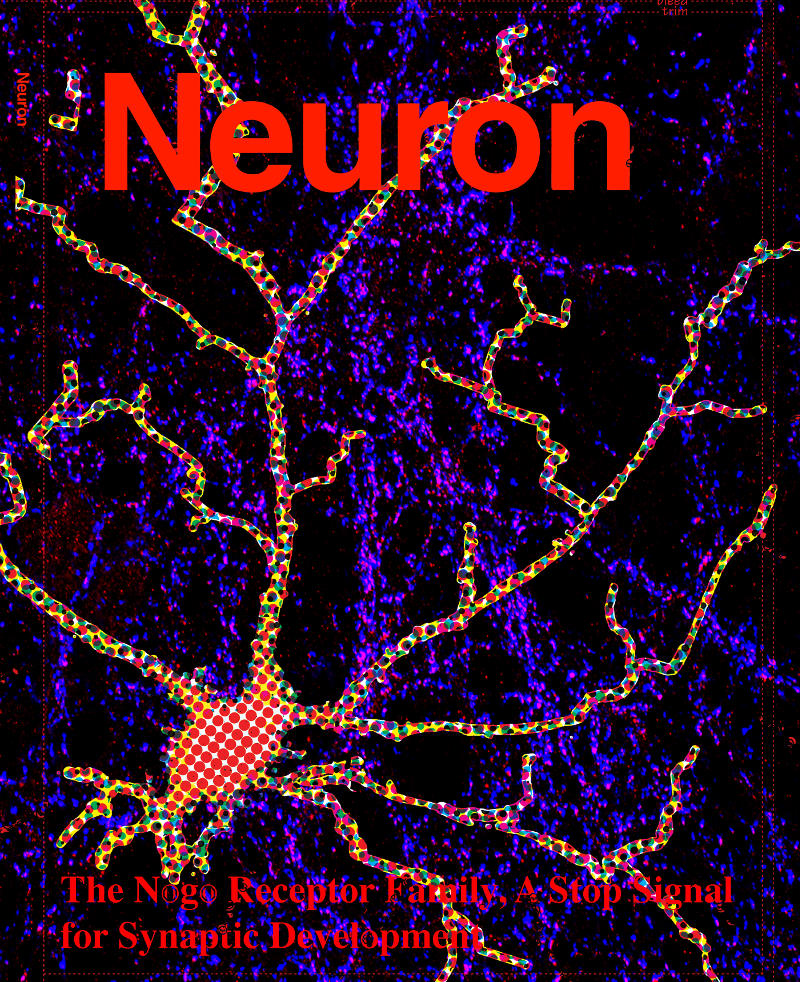
Neuronal development is characterized by a period of exuberant synaptic growth that is well studied. However, the mechanisms that restrict this process are less clear. Here we demonstrate that glycosylphosphatidylinositol-anchored cell-surface receptors of the Nogo Receptor family (NgR1, NgR2, and NgR3) restrict excitatory synapse formation. Loss of any one of the NgRs results in an increase in synapse number in vitro, whereas loss of all three is necessary for abnormally elevated synaptogenesis in vivo. We show that NgR1 inhibits the formation of new synapses in the postsynaptic neuron by signaling through the coreceptor TROY and RhoA. The NgR family is downregulated by neuronal activity, a response that may limit NgR function and facilitate activity-dependent synapse development. These findings suggest that NgR1, a receptor previously shown to restrict axon growth in the adult, also functions in the dendrite as a barrier that limits excitatory synapse number during brain development.
Zachary P. Wills, Caleigh Mandel-Brehm, Alan R. Mardinly, Alejandra E. McCord, Roman J. Giger, and Michael E. Greenberg
Cover art submission by Joh Axon (note: not chosen for the journal cover, but cool none the less!)
Cyclin E Constrains Cdk5 Activity to Regulate Synaptic Plasticity and Memory Formation
October 18, 2011 - Developmental Cell

Cyclin E is a component of the core cell cycle machinery, and it drives cell proliferation by regulating entry and progression of cells through the DNA synthesis phase. Cyclin E expression is normally restricted to proliferating cells. However, high levels of cyclin E are expressed in the adult brain. The function of cyclin E in quiescent, postmitotic nervous system remains unknown. Here we use a combination of in vivo quantitative proteomics and analyses of cyclin E knockout mice to demonstrate that in terminally differentiated neurons cyclin E forms complexes with Cdk5 and controls synapse function by restraining Cdk5 activity. Ablation of cyclin E led to a decreased number of synapses, reduced number and volume of dendritic spines, and resulted in impaired synaptic plasticity and memory formation in cyclin E-deficient animals. These results reveal a cell cycle-independent role for a core cell cycle protein, cyclin E, in synapse function and memory.
Junko Odajima, Zachary P. Wills, Yasmine M. Ndassa, Miho Terunuma, Karla Kretschmannova, Yan Geng, Sylwia Gawrzak, Isabel M. Quadros, Jennifer Newman, Manjusri Das, Marie E. Jecrois, Qunyan Yu, Na Li, Frederic Bienvenu, Stephen J. Moss, Michael E. Greenberg, Jarrod A. Marto, and Piotr Sicinski
Cover art by Helen Yang
Relevant Publications
Yang Bai, Miao Li, Yanmei Zhou, Lei Ma, Qian Qiao, Wanling Hu, Wei Li, Zachary Patrick Wills, and Wen-Biao Gan (2017)
Abnormal dendritic calcium activity and synaptic depotentiation occur early in a mouse model of Alzheimer’s disease
Mol Neurodegener. 2017; 12: 86.
MacDonald ML, Alhassan J, Newman JT, Richard M, Gu H, Kelly RM, Sampson AR, Fish KN, Penzes P, Wills ZP, Lewis DA, Sweet RA. (2017)
Selective Loss of Smaller Spines in Schizophrenia.
Am J Psychiatry. Jun 1;174(6):586-594.
Krivinko JM, Erickson SL, Abrahamson EE, Wills ZP, Ikonomovic MD, Penzes P, Sweet RA. (2017)
Kalirin reduction rescues psychosis-associated behavioral deficits in APPswe/PSEN1dE9 transgenic mice.
Neurobiol Aging. Jun;54:59-70.
Chung DW, Wills ZP, Fish KN, Lewis DA. (2017)
Developmental pruning of excitatory synaptic inputs to parvalbumin interneurons in monkey prefrontal cortex.
Proc Natl Acad Sci U S A. Jan 24;114(4):E629-E637.
Schulien AJ, Justice JA, Maio RD, Wills ZP, Shah NH, Aizenman E. (2016).
Zn2+ -Induced Ca2+ release via ryanodine receptors triggers calcineurin-dependent redistribution of cortical neuronal Kv2.1 K+ channels.
J Physiol. 2016 Mar 4.
Feng, B., Zhu, Y., La, J.-H., Wills, Z.P., Gebhart, G.P. (2015).
Experimental and computational evidence for an essential role of NaV1.6 in spike initiation at stretch-sensitive colorectal afferent endings.
J Neurophysiol 113(7):2618-34.PMID: 25652923
Shah, N., Schulien, A., Clemens, K., Aizenman, T., Hageman, T., Wills, Z., Aizenman, E. (2014).
Cyclin E1 Regulates Kv2.1 Channel Phosphorylation and Localization in Neuronal Ischemia.
J. Neurosci 34(12): 4326-31.
Margolis S.S., Salogiannis J., Lipton D.M., Mandel-Brehm C., Wills, Z.P., Mardinly A.R., Hu. L., Greer P.L., Bikoff J.B., Ho H.Y., Soskis M.J., Sahin M., Greenberg M.E. (2010)
EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation.
Cell, 143(3): 442-55. PMCID: PMC2967209
Wills, Z., Emerson, J., Rusch, J., Bikoff, J., Baum, B., Perrimon, N., Van Vactor, D. (2002).
A Drosophila Homolog of Cyclase-Associated Proteins Collaborates with the Abl Tyrosine Kinase to Control Midline Axon Pathfinding.
Neuron, 36: 611-622. PMID: 12441051
Wills, Z., Bateman, J., Korey, C.A., Van Vactor, D. (1999).
The Tyrosine Kinase Abl and its Substrate Enabled Collaborate with the Receptor Phosphatase Dlar to Control Motor Axon Guidance.
Neuron, 22: 301-312. PMID: 10069336
Wills, Z., Marr, L., Zinn, K., Goodman, C.S., Van Vactor, D. (1999).
Profilin and the Abl Tyrosine Kinase Are Required for Motor Axon Outgrowth in the Drosophila Embryo.
Neuron, 22: 291-299. PMID: 10069335
Kaufmann, N., Wills, Z.P., Van Vactor, D. (1998).
Drosophila Rac1 Controls Motor Axon Guidance.
Development, 125: 453-461. PMID: 9425140